Neuroscientist
Resume
15 Years of Experience
Experience
2023 - Present
Modendo-Inc, COSenior Neuroscience Engineer
Translating optical imaging technologies into tools for studying deep brain structures and developing CAD-based brain-optic interfaces.
2018 - 2023
University of Chicago, ILSenior Postdoctoral Researcher
Designed imaging tools to study sensorimotor cortex plasticity, including optical tools and comprehensive protocols for in vivo neuronal recordings.
2016 - 2018
Northwestern University, ILPostdoctoral Researcher
Investigated synaptic circuits and astrocyte-neuron interactions, developing open-source viral vectors and electrophysiological analysis tools.
2015-2016
Janssen Pharmaceuticals, BelgiumResearch Scientist
Developed human neuronal models to study Alzheimer’s disease, contributing to neurodegenerative disease research and advancing xenotransplantation techniques.
2010-2015
Université Libre de Bruxelles (ULB), BelgiumPhD Fellow in Neuroscience
In addition to my research, I collaborated with numerous teams, mentored students, provided training in lab techniques, presented, and published in peer-reviewed journals.
Education
2015
Université Libre de Bruxelles (ULB) & Collège de FrancePhD in Neuroscience
Research on neuroplasticity in the basal ganglia, exploring memory and pathological implications in Parkinson’s disease and cocaine addiction.
2009
Université Catholique de Louvain (UCL)MSc in Biomedical Science
Specialized in advanced fundamental research with a minor in neuroscience, focusing on experimental methods and foundational questions in bio-cellular and molecular sciences.
2007
Université Catholique de Louvain (UCL)BSc in Biomedical Sciences
Completed at the School of Medicine, with comprehensive studies in biology, chemistry, and human physiology, providing a solid foundation in biomedical sciences.
Core Expertise
- Neuroscience Research
- Data Analysis
- Scientific Visualization
- Experimental Design
- Cellular Biology
- Molecular Biology
- Client Collaboration
- Scientific/Grant Writing
- Project Management
- Cross-Disciplinary Collaboration
- Mentoring
- Public Speaking
Tools & Technologies
- Python
- MATLAB
- Fusion360
- Adobe Suite
- Jupyter Notebook
- Google Colab
- Microsoft Office Suite
- WordPress
AI Applications
- Data Analysis
- Deep Learning Architectures
- Predictive Analytics
- Model Optimization
- Workflow Automation
- Image Generation
- Visualization Enhancement
- Text Optimization
Professional Skills
- Scientific Writing
- Data Visualization
- Behavioral Statistics
- Optical Systems Development
- Pupillometry
- CAD/3D Printing
- Experimental Programming
Interests
- Outdoor Adventures
- Creative Problem Solving
- Knowledge Sharing
Languages
English: Bilingual
French: Native
My goal is to continue contributing to neuroscience and engineering by translating research into impactful solutions, sharing innovation through scientific writing, grant development, and multidisciplinary collaboration.
Accomplishments










Testimonials
"Dr Lambot developed a novel behavioral paradigm to study natural touch in rodents. This involved engineering novel instruments to evoke natural touch and the application of advance machine learning algorithms to quantify natural touch. Of course, Dr Lambot is a neuroscientist so she combined this new set of instruments with brain recordings. [...] The breadth of her work is highly unusual and demonstrates a tremendous intellect."
"Laurie's collaborative leadership has consistently delivered high-impact results in understanding neuronal excitability and synaptic plasticity. Her work exemplifies the innovative drive needed to tackle critical challenges in neuroscience. [...] Her leadership and vision make her a key figure in modern neuroscience"

David Gall, PhD
"Dr. Lambot is an excellent young scientist whose extensive record of scientific productivity and expertise in neuroplasticity mechanisms has greatly enriched our editorial team. Her inclusion was a unanimous decision, reflecting her outstanding scientific reputation."

Arianna Maffei, PhD
Specialty Chief Editor at Frontiers in Cellular Neuroscience and Neurophysiology
"Dr. Lambot’s contributions were essential in establishing a novel chimeric model for Alzheimer’s disease, allowing the analysis of genetic backgrounds and mutations. Her innovative approach offers a unique opportunity to revolutionize Alzheimer’s research."

Pierre Vanderhaeghen, MD, PhD
Full Professor and Group Leader at the VIB Centre for Brain and Disease Research
"She was able to explore new ideas with an open mind and transfer concepts from neuroscience to the reproductive system field, making her contributions invaluable to our research. I believe that she has the potential to leverage her extensive knowledge and skills in neuroscience to catalyze progress in other research domains."

Pascale Lybaert, PhD, DVM
Professor at the Research Laboratory on Human Reproduction at the ULB
"Her ingenious approaches to overcoming imaging distortions have set a new standard for precision in neuronal imaging. Her ability to innovate and lead in solving complex problems positions her as a future leader in neuroscience technology development."
"Dr. Lambot’s groundbreaking research has demonstrated that memories can be artificially implanted without natural stimuli, leading to significant implications for the study of memory and its associated disorders. Her work holds great promise for developing novel treatments for memory-related conditions such as dementia or Alzheimer’s disease."

Christian Hansel, PhD
"Laurie Lambot’s contributions to the development of custom viral vectors demonstrate her leadership in advancing global research tools. Her forward-thinking approach ensures that her work will continue to shape the future of neuroscience."

Gordon MG Shepherd, MD, PhD
"Her remarkable ion channel research showcases her innovative approach to complex problems. Dr. Lambot’s leadership and vision will undoubtedly inspire transformative discoveries in neuroscience."

Enrique Balderas-Angeles, PhD
Research Associate for the Cardiovascular Research and Training Institute at the University of Utah
"Laurie is not just a scientist; she’s a leader who understands how to translate innovative research into commercially viable products. Her work at Modendo is poised to shape the future of medical technology, driving improvements in patient care and clinical practice efficiency."

Tim Morrissey, PhD
Investor, Drive Capital Former CEO & Co-founder, Artimus Robotics
"Her presentation was impressive, not only for its technological innovation but also for Laurie’s ability to clearly communicate the implications of this work for both research and clinical applications. The audience of investors and industry experts was highly engaged and enthusiastic about the potential of this cutting-edge technology."
Portfolio
My Works
My Career – Chapter 1: Synaptic Plasticity
Animal Models, Brain ImagingMy Career – Chapter 2: Non-Synaptic Plasticity
Career & InnovationMy Career – Chapter 3: Friedreich’s Ataxia
Career & InnovationMy Career – Chapter 4: Alzheimer’s Disease
Career & InnovationMy Career -Chapter 5: Driving Innovation and Collaboration
Career & InnovationDigital Fabrication for Multimodal Recording, Including Brain Recording
Animal Models, Brain Imaging, Engineering & DesignInnovative Contention Jacket Design for Marmoset Neural Research
Animal Models, Engineering & DesignCustom Implant System for Head-Restrained Neural Recordings: The Head Attachment Tool (HAT)
Engineering & Design3D Visualization of a Striatopallidal Neuron Dendrite – Confocal Imaging and Deconvolution
Brain ImagingHow We Map Motor Control in Mice with Laser Precision
Animal ModelsMouse Pupil Dynamics and Whisking Behavior with DLC
Animal ModelsTracking Mouse Locomotion Using DeepLabCut in Open Field Test
Animal ModelsVisualizing Cortical Oxygenation Dynamics in Response to Whisker Stimulation in Mice
Animal Models, Brain ImagingPupillary Dynamics and Ocular Behavior in a Head-Restrained Mouse Under Constant Illumination
Animal ModelsWhisker Tracking During Simulated Tunnel Running in Head-Fixed Mice
Animal ModelsIlluminating Cortico-Spinal Neuronal Pathways in the Mouse Brain
Brain ImagingImpediments in Learning Complex Motor Coordination for Pellet Retrieval from Challenging Locations
Animal Models‘Parasite’ Movement Phenomenon in Motor Skill Acquisition
Animal ModelsAutomated Push Syringe System for Behavioral Training in Mice
Engineering & DesignMouse Pupil Dynamics During Multimodal Imaging
Animal ModelsEditorial & Peer-Review Roles
Cellular Neurophysiology focuses on the mechanisms by which neurons and glial cells generate, regulate, and integrate electrical and biochemical signals at the cellular and sub-cellular levels. The journal publishes work spanning ion channel function, synaptic transmission, intracellular signaling, and neuron–glia interactions, with relevance to both fundamental neuroscience and disease-related dysfunction.
As a Review Editor, I contribute to the evaluation of manuscripts addressing cellular signaling, circuit integration, and experimental approaches that link molecular mechanisms to physiological function.
→ Journal website.
Motivation and Reward covers neural mechanisms underlying goal-directed behavior, reinforcement learning, decision-making, and affective processing. The journal emphasizes integrative approaches combining systems neuroscience, behavioral paradigms, computational models, and translational perspectives relevant to psychiatric and neurological disorders.
In this role, I contribute as an expert peer reviewer, evaluating studies focused on cortico-striatal circuits, neuromodulatory systems, and experimental designs that link neural activity to behavior.
→ Journal website.
Neurophotonics is an interdisciplinary journal dedicated to the development and application of optical technologies for studying the nervous system. It publishes advances in optical imaging, light-based neural modulation, computational optics, and instrumentation enabling high-resolution interrogation of neural structure and function across scales.
In this role, I assess submissions at the interface of optical engineering and neuroscience, with particular attention to methodological rigor, innovation in imaging strategies, and relevance to in vivo and translational research.
→ Journal website.
Publications
Toth AB, Hori K, Novakovic MM, Bernstein NG, Lambot L, Prakriya M. 2019. May 21;12(582). doi: 10.1126/scisignal.aaw5450.
The Big Picture: We discovered the critical role of CRAC channels in astrocytes—the most abundant non-neuronal cells in the central nervous system—in neuron communication. These channels are essential for releasing molecules that regulate neuron activity. Disabling them reduced the astrocytes’ ability to release signaling molecules, diminishing neuron activity. Our findings open new avenues for modulating brain cell activity, potentially impacting brain function and neurological disorder understanding. Article available here.
Abstract: Astrocytes are the major glial subtype in the brain and mediate numerous functions ranging from metabolic support to gliotransmitter release through signaling mechanisms controlled by Ca2+ Despite intense interest, the Ca2+ influx pathways in astrocytes remain obscure, hindering mechanistic insights into how Ca2+ signaling is coupled to downstream astrocyte-mediated effector functions. Here, we identified store-operated Ca2+ release-activated Ca2+ (CRAC) channels encoded by Orai1 and STIM1 as a major route of Ca2+ entry for driving sustained and oscillatory Ca2+ signals in astrocytes after stimulation of metabotropic purinergic and protease-activated receptors. Using synaptopHluorin as an optical reporter, we showed that the opening of astrocyte CRAC channels stimulated vesicular exocytosis to mediate the release of gliotransmitters, including ATP. Furthermore, slice electrophysiological recordings showed that activation of astrocytes by protease-activated receptors stimulated interneurons in the CA1 hippocampus to increase inhibitory postsynaptic currents on CA1 pyramidal cells. These results reveal a central role for CRAC channels as regulators of astrocyte Ca2+ signaling, gliotransmitter release, and astrocyte-mediated tonic inhibition of CA1 pyramidal neurons.
Yamawaki N, Li X, Lambot L, Ren LY, Radulovic J, Shepherd GMG. 2019 Apr;22(4):618-626. doi: 10.1038/s41593-019-0355-x.
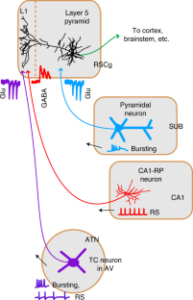
The Big Picture: We identified a unique population of GABAergic neurons linking the hippocampus to the retrosplenial cortex, crucial for cognitive functions. These neurons inhibit specific targets in the cortex, balancing excitatory inputs and affecting memory processes. This finding sheds light on the intricate mechanisms of memory encoding. Article available here.
Abstract: Hippocampus, granular retrosplenial cortex (RSCg), and anterior thalamic nuclei (ATN) interact to mediate diverse cognitive functions. To identify cellular mechanisms underlying hippocampo-thalamo-retrosplenial interactions, we investigated the potential circuit suggested by projections to RSCg layer 1 (L1) from GABAergic CA1 neurons and ATN. We find that CA1→RSCg projections stem from GABAergic neurons with a distinct morphology, electrophysiology, and molecular profile. Their long-range axons inhibit L5 pyramidal neurons in RSCg via potent synapses onto apical tuft dendrites in L1. These inhibitory inputs intercept L1-targeting thalamocortical excitatory inputs from ATN to coregulate RSCg activity. Subicular axons, in contrast, excite proximal dendrites in deeper layers. Short-term plasticity differs at each connection. Chemogenetically abrogating CA1→RSCg or ATN→RSCg connections oppositely affects the encoding of contextual fear memory. Our findings establish retrosplenial-projecting CA1 neurons as a distinct class of long-range dendrite-targeting GABAergic neuron and delineate an unusual cortical circuit specialized for integrating long-range inhibition and thalamocortical excitation.
Espuny-Camacho I, Arranz AM, Fiers M, Snellinx A, Ando K, Munck S, Bonnefont J, Lambot L, Corthout N, Omodho L, Vanden Eynden E, Radaelli E, Tesseur I, Wray S, Ebneth A, Hardy J, Leroy K, Brion JP, Vanderhaeghen P, De Strooper B. 2017 Mar 8;93(5):1066-1081.e8. doi: 10.1016/j.neuron.2017.02.001.
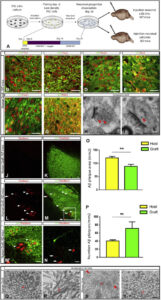
The Big Picture: We have developed a chimeric model for Alzheimer’s Disease (AD) by transplanting human stem cell-derived neuronal precursors into mouse brains. This model showcases the degeneration of human neurons amidst AD. Through this human-mouse hybrid model, we observed changes in gene expression affecting memory, cognition, and synaptic functions, providing a novel platform for exploring human-specific aspects and potential treatments for AD. Article available here.
Abstract: Human pluripotent stem cells (PSCs) provide a unique entry to study species-specific aspects of human disorders such as Alzheimer’s disease (AD). However, in vitro culture of neurons deprives them of their natural environment. Here we transplanted human PSC-derived cortical neuronal precursors into the brain of a murine AD model. Human neurons differentiate and integrate into the brain, express 3R/4R Tau splice forms, show abnormal phosphorylation and conformational Tau changes, and undergo neurodegeneration. Remarkably, cell death was dissociated from tangle formation in this natural 3D model of AD. Using genome-wide expression analysis, we observed upregulation of genes involved in myelination and downregulation of genes related to memory and cognition, synaptic transmission, and neuron projection. This novel chimeric model for AD displays human-specific pathological features and allows the analysis of different genetic backgrounds and mutations during the course of the disease.
Lambot L, Gall D. 2016 8-9;32(8-9):768-70. doi: 10.1051/medsci/20163208026.
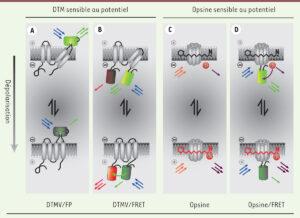
The Big Picture: In this publication I review the recent advances in optical imaging with voltage indicators, a key development for high-resolution analysis of brain activity. These indicators now accurately capture individual and rapid neuron firing sequences. When combined with optogenetics, this method paves the way for comprehensive, all-optical electrophysiology studies. Article available here.
Abstract: Optical imaging of voltage indicators is a promising approach for detecting the activity of neuronal circuits with high spatial and temporal resolution. In this context, genetically encoded voltage indicators, combining genetic targeting and optical readout of transmembrane voltage, represent a technological breaktrough that will without doubt have a major impact in neuroscience. However, so far the existing genetically encoded voltage indicators lacked the capabilities to detect individual action potentials and fast spike trains in live animals. Here, we present a novel indicator allowing high-fidelity imaging of individual spikes and dentritic voltage dynamics in vivo. Used in combination with optogenetics, which allows to manipulate neuronal activity, this opens the possibility of an all-optical electrophysiology.
Lambot L, Chaves Rodriguez E, Houtteman D, Li Y, Schiffmann SN, Gall D, de Kerchove d’Exaerde A. 2016 May 4;36(18):4976-92. doi: 10.1523/JNEUROSCI.2717-15.2016.
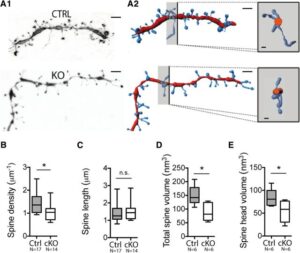
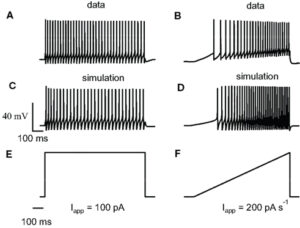
The Big Picture: My research reveals the crucial role of NMDA receptors in striatopallidal neurons, essential for coordinating behaviors like habit formation, locomotion, and learning. Eliminating these receptors leads to significant behavioral changes, highlighting their importance in the basal ganglia’s operations. This insight not only advances our understanding of motor and learning processes but also implies potential impacts on Parkinson’s disease and addiction, suggesting new paths for treatments. Article available here.
Abstract: The basal ganglia (BG) control action selection, motor programs, habits, and goal-directed learning. The striatum, the principal input structure of BG, is predominantly composed of medium-sized spiny neurons (MSNs). Arising from these spatially intermixed MSNs, two inhibitory outputs form two main efferent pathways, the direct and indirect pathways. Striatonigral MSNs give rise to the activating, direct pathway MSNs and striatopallidal MSNs to the inhibitory, indirect pathway (iMSNs). BG output nuclei integrate information from both pathways to fine-tune motor procedures and to acquire complex habits and skills. Therefore, balanced activity between both pathways is crucial for harmonious functions of the BG. Despite the increase in knowledge concerning the role of glutamate NMDA receptors (NMDA-Rs) in the striatum, understanding of the specific functions of NMDA-R iMSNs is still lacking. For this purpose, we generated a conditional knock-out mouse to address the functions of the NMDA-R in the indirect pathway. At the cellular level, deletion of GluN1 in iMSNs leads to a reduction in the number and strength of the excitatory corticostriatopallidal synapses (video 1). The subsequent scaling down in input integration leads to dysfunctional changes in BG output, which is seen as reduced habituation, delay in goal-directed learning, lack of associative behavior, and impairment in action selection or skill learning (video 2 & video 3). The NMDA-R deletion in iMSNs causes a decrease in the synaptic strength of striatopallidal neurons, which in turn might lead to a imbalanced integration between direct and indirect MSN pathways, making mice less sensitive to environmental change. Therefore, their ability to learn and adapt to the environment-based experience was significantly affected.
Piccart E, De Backer JF, Gall D, Lambot L, Raes A, Vanhoof G, Schiffmann S, D’Hooge R. 2014 Jul 15;268:48-54. doi: 10.1016/j.bbr.2014.03.016.
The Big Picture: We uncovered the essential role of the enzyme PDE10A in the brain’s reward system, demonstrating that it is crucial for processing and learning from rewarding stimuli. Mice lacking PDE10A experienced significant challenges in recognizing rewarding cues, mirroring aspects of schizophrenia. Our work not only deepens understanding but also paves the way for novel approaches to addressing motivational and cognitive disorders. Article available here.
Abstract: The striatum is the main input structure to the basal ganglia and consists mainly out of medium spiny neurons. The numerous spines on their dendrites render them capable of integrating cortical glutamatergic inputs with a motivational dopaminergic signal that originates in the midbrain. This integrative function is thought to underly attribution of incentive salience, a process that is severely disrupted in schizophrenic patients. Phosphodiesterase 10A (PDE10A) is located mainly to the striatal medium spiny neurons and hydrolyses cAMP and cGMP, key determinants of MSN signaling. We show here that genetic depletion of PDE10A critically mediates attribution of salience to reward-predicting cues, evident in impaired performance in PDE10A knockout mice in an instrumentally conditioned reinforcement task. We furthermore report modest impairment of latent inhibition in PDE10A knockout mice, and unaltered prepulse inhibition. We suggest that the lack of effect on PPI is due to the pre-attentional nature of this task. Finally, we performed whole-cell patch clamp recordings and confirm suggested changes in intrinsic membrane excitability. A decrease in spontaneous firing in striatal medium spiny neurons was found. These data show that PDE10A plays a pivotal role in striatal signaling and striatum-mediated salience attribution.
Hick A, Wattenhofer-Donzé M, Chintawar S, Tropel P, Simard JP, Vaucamps N, Gall D, Lambot L, André C, Reutenauer L, Rai M, Teletin M, Messaddeq N, Schiffmann SN, Viville S, Pearson CE, Pandolfo M, Puccio H. 2013 May;6(3):608-21. doi: 10.1242/dmm.010900.
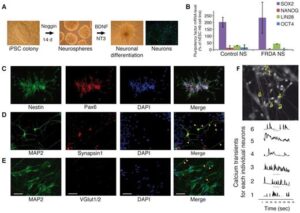
The Big Picture: We’ve developed neurons from patient-derived iPSCs to explore mitochondrial dysfunction in Friedreich’s ataxia (FRDA). These neurons demonstrate critical mitochondrial issues, mirroring the disease’s neurodegenerative effects. This breakthrough serves as a vital model for studying FRDA’s mitochondrial challenges and informed a clinical trial targeting these defects. Article available here.
Abstract: Friedreich’s ataxia (FRDA) is a recessive neurodegenerative disorder commonly associated with hypertrophic cardiomyopathy. FRDA is due to expanded GAA repeats within the first intron of the gene encoding frataxin, a conserved mitochondrial protein involved in iron-sulphur cluster biosynthesis. This mutation leads to partial gene silencing and substantial reduction of the frataxin level. To overcome limitations of current cellular models of FRDA, we derived induced pluripotent stem cells (iPSCs) from two FRDA patients and successfully differentiated them into neurons and cardiomyocytes, two affected cell types in FRDA. All FRDA iPSC lines displayed expanded GAA alleles prone to high instability and decreased levels of frataxin, but no biochemical phenotype was observed. Interestingly, both FRDA iPSC-derived neurons and cardiomyocytes exhibited signs of impaired mitochondrial function, with decreased mitochondrial membrane potential and progressive mitochondrial degeneration, respectively. Our data show for the first time that FRDA iPSCs and their neuronal and cardiac derivatives represent promising models for the study of mitochondrial damage and GAA expansion instability in FRDA.
Bischop DP, Orduz D, Lambot L, Schiffmann SN, Gall D. 2012;5:78. doi: 10.3389/fnmol.2012.00078. eCollection 2012.
The Big Picture: We modeled the activity of striatal fast-spiking interneurons (FSIs) and discovered how parvalbumin, a calcium-binding protein, influences their excitability. Our study sheds light on the role of parvalbumin in regulating brain communication and processing, suggesting its significance in neural circuit function. Article available here.
Abstract: Calcium binding proteins, such as parvalbumin (PV), are abundantly expressed in distinctive patterns in the central nervous system but their physiological function remains poorly understood. Notably, at the level of the striatum, where PV is only expressed in the fast-spiking (FS) interneurons. FS interneurons form an inhibitory network modulating the output of the striatum by synchronizing medium-sized spiny neurons (MSN). So far the existing conductance-based computational models for FS neurons did not allow the study of the coupling between PV concentration and electrical activity. In the present paper, we propose a new mathematical model for the striatal FS interneurons that includes apamin-sensitive small conductance Ca(2+)-dependent K(+) channels (SK) and the presence of a calcium buffer. Our results show that a variation in the concentration of PV can modulate substantially the intrinsic excitability of the FS interneurons and therefore may be involved in the information processing at the striatal level.
Presentations
L. LAMBOT, S. E. MONTGOMERY, O. TZANG, R. PIESTUN, A. CARAVACA AGUIRRE.
@ SfN2024, Chicago, IL, USA.
Abstract: Over the past decade, the notion that isolated neuron populations support cognition and behavior has shifted towards an understanding that complex behaviors emerge from interactions within anatomically connected, specialized networks. Advances in brain imaging techniques have expanded the tools available to explore the functional organization of these networks. However, traditional methods face significant limitations, damaging the tissue, disrupting synaptic connections, and destroying the brain’s integral circuit architecture. Here, we introduce a commercial prototype that employs wavefront shaping through a hair-thin probe to resolve cellular and sub-cellular structures with spatial, sub-micron precision. Utilizing computational optics, this system generates arbitrary, digitally programmed light patterns at the distal tip of the probe at a speed of 23 kHz. Real-time high-resolution 2D and 3D images are acquired via a user-friendly interface. Our ultrathin endomicroscope is compact, mobile, and well-suited for head-fixed recordings in rodents while supporting multiple laser inputs. Endoscopic imaging experiments conducted in vitro with phantom brains containing fluorescent beads, ex vivo brain slices, as well as in vivo in mice demonstrate single-cell precision across multiple imaging planes, including the capability to visualize somatic and dendritic compartments with minimal tissue impact. Multi-color imaging through the same fiber probe facilitates the visualization of fluorescent reporters and the acquisition of neuronal activity of distinct ensembles using genetically encoded calcium indicators. Current efforts in developing this technology focus on single-cell optogenetic modulation and multi-site recordings during both anesthetized and awake, behaving animal experiments. This ultrathin endomicroscopy opens new avenues for both structural and functional imaging, providing unprecedented access to deep brain structures in vivo and the capability to map and manipulate cells across intact neural networks with minimal damage.
Lambot L., Montgomery S.E., Tzang O, Moser C., Psaltis D., Piestun R, Caravaca-Aguirre A.,
Modendo Inc., Boulder, CO; EPFL, Lausanne, CH; University of Colorado Boulder, CO.
@NINDS 2024, Chicago, IL, USA.
Abstract: Modendo is at the forefront of developing novel ultrathin endomicroscopes, addressing critical challenges in neuroscience. These cutting-edge instruments provide high-resolution optical imaging and photostimulation capabilities for regions of the brain that were previously difficult or impossible to access.
Our technological innovation lies in our use of ultrathin imaging probes, with the width of a human hair, which enable observation of large sets of interacting neurons in vivo with minimal damage, essential for understanding the circuitry function and dysfunction of the brain and facilitating the exploration and visualization of brain activity in complex and delicate regions, such as the brainstem and olfactory bulb, with minimal disruption to neural structures.
Modendo endomicroscope system (Figure) can accommodate multiple probes simultaneously allowing researchers to probe multiple brain regions during the same experiment. The integration of multiple probes within a single system allows for simultaneous imaging, or imaging and stimulation in different brain regions, providing a holistic view of brain function that has been elusive with previous technologies. This capability offers unprecedented opportunities to study the brain’s connectome by correlating high resolution imaging (Figure), precise neural stimulation and activity recording across various brain areas. Understanding these complex networks can lead to significant insights into the functional disparities between healthy and diseased brains, potentially guiding the development of improved treatments for neurological disorders such as Parkinson’s disease, schizophrenia, and epilepsy. Additionally, our system allows for 3D imaging with micrometer resolution, enabling comprehensive studies of neural connectivity and function.
This ability to penetrate deep brain regions with minimal tissue damage while maintaining high-resolution imaging capabilities represents a significant advancement in neural research. This innovation supports the study of fundamental questions regarding the organization of neural circuits, how small-scale networks compute, and how they contribute to complex behaviors.
One of the unique advantages of Modendo’s technology is its ability to deliver precise light patterns for both photostimulation and potentially photo-ablation, enabling targeted modulation of neural activity. This versatility opens doors to a wide range of applications, from accurately targeting and modulating neural circuits to the selective removal of specific cells.
Modendo’s multiprobe technology is set to transform neuroscience research by providing a powerful tool for in vivo studies of brain structure and function. We invite you to explore the diverse applications of our ultra-thin multiprobe endomicroscope and engage in discussions about how this technology can advance your research.
Pioneering neuroscience advancements: Modendo’s ultra-thin probes for therapeutic insights [Poster].
Brain Initiative 2023
Small Business Program (SBIR & STTR)
Poster #47
Laurie Lambot
Abstract: Pioneering neuroscience advancements: Modendo’s ultra-thin probes for therapeutic insights Modendo is leading the development of endomicroscopes equipped with ultra-thin, minimally-invasive probes. This cutting-edge instrumentation addresses a critical need within the neuroscience community, advancing our understanding of the brain. Our technology enables researchers to explore and visualize brain activity in previously challenging regions, such as the brainstem and olfactory bulb, with minimal impact on neural structures. The ability to insert multiple imaging probes into intricate brain areas offers a unique opportunity to expand our knowledge of the brain’s connectome. By correlating neural stimulation and activity across various brain regions, we aim to uncover the complex network of connections governing brain function. This advancement promises valuable insights into the functional disparities between healthy and diseased brains, potentially leading to improved treatments and interventions. Modendo’s innovation extends beyond imaging. Its comprehensive system solution enables projection of precise laser patterns for both photo-stimulation and selective energy delivery. This versatile approach opens opportunities for a wide range of applications, from accurately targeting and modulating neural activity to the selective removal of problematic tissues. We invite you to engage in discussions with us about the diverse applications of our ultra-thin probe endomicroscope.
BioFrontiers Advanced Light Microscopy Core at University of Colorado, February 9th, 2023, Boulder, CO, USA.
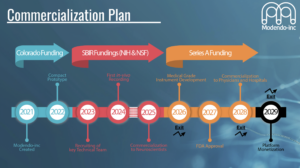
I presented this deck at the Ascent Deep Tech Accelerator Investor Showcase in June 2024. The presentation focused on my work developing imaging tools for deep brain structures, including Modendo’s progress with the Ultrathin Endoscope (UTE) for cellular imaging. The event provided an opportunity to share insights with investors and industry leaders while receiving valuable feedback to refine future projects. Here is the link to my Pitch Deck.

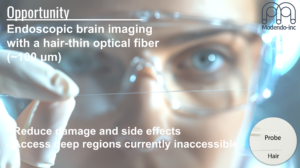
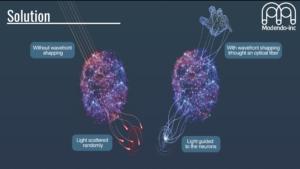
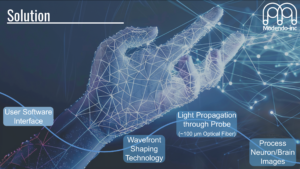

The study of ion channels gatekeepers of life (Biophysical Society), May 2nd. 2022.
Society for Neuroscience, 2017, Nov 14th, Washington D.C., USA.
Society for Neuroscience, Nov 13th, 2017, Washington D.C., USA.
10th FENS Forum of Neuroscience, July 4th, 2016, Copenhagen, Denmark.
Pulic PhD Defense, August 19th, 2015, Brussels, Belgium.
IAP 7 ELECXITE Annual
meeting, June 3rd, 2015, Liège, Belgium.
11th National Congress of the Belgian Society for Neuroscience, May 22nd, 2015, Mons, Belgium.
14ème Journée des Doctorants, December 18th, 2014, Brussels, Belgium.
Fondation Médicale Reine
Elisabeth, date, Royal Palace of Brussels, Brussels, Belgium, 2014.
Belgian Society of Physiology and Pharmacology, October 17th, 2014, Brussels, Belgium.
IAP Annual meeting, October 7th, 2014, Leuven, Belgium.
IAP Annual meeting, October 7th, 2014, Leuven, Belgium.
Autumn Meeting of the Belgian Society of Fundamental and Clinical Physiology and Pharmacology, August 26th, 2014, Leuven, Belgium.
Spring meeting 2014 of the Belgian Physiological Society, May 22nd, 2014, Brussels, Belgium.
58th annual Meeting of the BiophySical Society, February 16th, 2014, San Francisco, California, USA.
IAP 7 ELECXITE Annual meeting, April 26th, 2013, Brussels, Belgium.
FRIA doctoral grant (Fund for Research Training in Industry and Agriculture), September 26th, 2010, Brussels, Belgium.
6th European Congress on Tropical Medicine and International Health, September 6–10, 2009, Verona, Italy. Link here.
This epidemiological project evaluated the prevalence of iodine deficiency disorders in the Kapolowe district (Katanga Province, DRC) through field-based clinical examinations and community screening. Working in collaboration with the University of Lubumbashi, the team conducted structured assessments of goitre, collected biological samples for iodine status evaluation, and coordinated logistics to ensure sample integrity across multiple health centers. The project also included community outreach to improve awareness of iodine intake and the use of iodized salt. The resulting data informed a peer-reviewed abstract presented at the 6th European Congress on Tropical Medicine and International Health and published in Tropical Medicine & International Health (Vol. 14, p. 223, 2009).
Peer-Reviewed Abstracts
- Poster: PSTR430 – Optogenetic Tools
Location: MCP Hall A
Time: Wednesday, October 9, 2024, 8:00 AM – 12:00 PM
Program # / Poster #: PSTR430.28 / Y27
Topic: I.08. Methods to Modulate Neural Activity
Support:
- NIH Award 5R43NS127710
- NSF Award 2212906
- OEDIT Advance Industries Grant CTGG1 2024-2090
Title:
Next-generation ultrathin endomicroscopy for high-resolution, minimally invasive imaging of deep brain structures
Authors:
L. Lambot¹, S. E. Montgomery¹, O. Tzang²,³, R. Piestun², A. Caravaca Aguirre¹
¹University of Colorado Boulder
²Modendo-Inc., Boulder, CO
³Modendo, Boulder, CO
Abstract:
Over the past decade, our understanding of cognition and behavior has evolved from isolated neuronal functions to a recognition of their emergence from interactions within anatomically connected, specialized networks. Advanced brain imaging techniques have significantly expanded our ability to explore these functional networks, yet many traditional methods damage tissue, disrupt synaptic connections, and compromise the brain’s circuit architecture.
We introduce a cutting-edge prototype ultrathin endomicroscope, leveraging wavefront shaping through a hair-thin probe to achieve spatial, sub-micron precision for cellular and sub-cellular imaging. This system, employing computational optics, generates programmable light patterns at 23 kHz and provides real-time high-resolution 2D and 3D imaging through an intuitive user interface.
The device is compact, mobile, and optimized for head-fixed recordings in rodents, supporting multiple laser inputs. Validation experiments in vitro (phantom brains with fluorescent beads), ex vivo (brain slices), and in vivo (mouse models) demonstrated precision imaging of somatic and dendritic compartments with minimal tissue disruption. Multi-color imaging allows simultaneous visualization of fluorescent reporters and neuronal activity using genetically encoded calcium indicators.
Current advancements include integrating single-cell optogenetic modulation and multi-site recordings during awake and anesthetized animal studies. This technology represents a breakthrough in structural and functional imaging, offering unparalleled access to deep brain structures and enabling precise mapping and manipulation of intact neural networks with minimal damage.
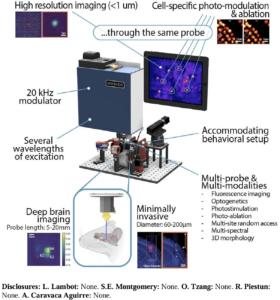
Key Features:
- High-resolution imaging at cellular and sub-cellular levels
- Compact and mobile design for in vivo studies
- Multi-color imaging for distinct neuronal activity visualization
Minimal tissue damage with advanced wavefront shaping technology
Poster: Session 532 – Software Tools I
Poster Number: 532.21 / WW38
Title:
WAVESURFER: A Freely Available Data Acquisition, Signal Generation, and Device Control Software Package for Experimental Neuroscience
Conference: Neuroscience 2017
Date and Time: Tuesday, November 14, 2017, 8:00 AM – 12:00 PM
Presenter at Poster: 8:00 AM – 9:00 AM
Location: Halls A-C
Grant Support: NIH Grant NS086549
Authors:
J.M. Barrett, A.L. Taylor, L.S. Lambot, X. Li, H. Inagaki, K. Svoboda, B. Kimmel, G.M.G. Shepherd
- Department of Physiology, Feinberg School of Medicine, Northwestern University, Chicago, IL
- Janelia Research Campus, Howard Hughes Medical Institute, Ashburn, VA
- Vidrio Technologies, Ashburn, VA
Abstract:
Modern neuroscience experiments rely on integrating various hardware components, each with distinct interfaces and control software, to collect diverse signals (e.g., electrophysiological, optical, behavioral) and generate stimuli across multiple modalities (e.g., electrical, optical, auditory, tactile). Writing custom experimental control routines is one approach but is time-intensive, requires programming expertise, and lacks flexibility when experimental configurations change.
WaveSurfer (wavesurfer.janelia.org) addresses these challenges by providing a flexible and user-friendly software solution. Building on features from Ephus (Suter et al., 2010), WaveSurfer enables coordinated electrophysiological recording and stimulation, acquisition and generation of arbitrary 1D analog signals, and triggering of external devices. Its intuitive interface allows on-the-fly reconfiguration of experiments, while custom MATLAB scripts can be integrated for real-time analysis or sophisticated closed-loop experiments.
WaveSurfer is compatible with National Instruments X series data acquisition boards and a range of patch-clamp amplifiers, with optimized integration for Axon and Heka amplifiers. Additionally, WaveSurfer integrates with ScanImage (Vidrio) for laser scanning microscopy and photostimulation, including two-photon imaging.
Applications and Capabilities:
- Slice Electrophysiology: Delivers arbitrary voltage or current stimuli, supports multi-wavelength optical stimulation with LEDs or lasers.
- In Vivo Imaging: Used for transcranial calcium imaging combined with Micro-Manager (Edelstein et al., 2014) and Retiga 2000DC cameras (QImaging).
- Optogenetics: Synchronizes optical recordings with piezoelectric tactile stimulation or delivers respiration-locked optogenetic stimulation by monitoring breathing.
WaveSurfer’s flexibility and extensibility make it a robust solution for diverse experimental setups. Originally designed for slice-based electrophysiology, it is now utilized across various neuroscientific experiments in our labs.
Key Features:
- Simplified user interface for flexible experimental control
- Integration with MATLAB for real-time analysis and customization
- Compatibility with multiple amplifiers and data acquisition systems
- Seamless integration with optical imaging tools for advanced microscopy
WaveSurfer is a powerful, accessible tool that streamlines experimental workflows, enhancing research capabilities in neuroscience.
Poster: Session 407 – Cortical Planning and Execution: Animal Neurophysiology
Poster Number: 407.12 / LL13
Title:
Transcranial Laser Scanning Photostimulation and Video Motion Tracking for Optogenetic Cortical Motor Mapping of Mouse Corticospinal Neurons
Conference: Neuroscience 2017
Date and Time: Monday, November 13, 2017, 1:00 PM – 5:00 PM
Presenter at Poster: 4:00 PM – 5:00 PM
Location: Halls A-C
Grant Support: NIH / NINDS Grant # NS061963
Authors:
L. Lambot, J.M. Barrett, X. Li, G.M. Shepherd
- Department of Physiology, Feinberg School of Medicine, Northwestern University, Chicago, IL
Abstract:
Motor mapping, a technique to stimulate cortical locations and measure evoked movements, is a foundational tool for studying motor cortical areas. However, traditional electrophysiological mapping methods are limited by poor spatial resolution and lack of cell type specificity. Light-based mapping (LBM; Ayling et al., 2009) addresses these issues, offering precise, cell-specific insights.
We present a variant of LBM combining transcranial laser scanning photostimulation (tcLSPS) with video motion tracking (VMT) using low-cost cameras. This tcLSPS-VMT approach enables detailed mapping of cortically evoked movements through selective photostimulation of corticospinal neurons expressing channelrhodopsin-2 (ChR2).
To achieve specificity, corticospinal neurons were labeled with ChR2 via cervical spinal cord injections of rAAV2-retro-Syn-ChR2-GFP (courtesy of Alla Karpova, Janelia), a retrogradely transported AAV (Tervo et al., 2016). Ketamine-anesthetized, head-fixed mice underwent cortical stimulation via tcLSPS, employing blue laser beams controlled by scanning galvanometers (Thorlabs) and acousto-optical modulators for power regulation.
Evoked movements were captured with dual USB3 CMOS video cameras (Chameleon3, FLIR) recording at 100–250 fps. Laser parameters and video capture were managed using LabView. Offline analysis was performed with a MATLAB-based motion tracking algorithm, utilizing ROI-based, 2D cross-correlation techniques to measure paw movements from video data.
Key Results:
- Demonstrated the efficacy of tcLSPS-VMT for detecting and characterizing limb movements evoked by cortical stimulation.
- Highlighted that selective activation of corticospinal neurons can reliably evoke specific motor responses.
This tcLSPS-VMT technique offers a cost-effective, scalable approach for precise, optogenetic motor mapping, providing valuable insights into the functional organization of motor circuits.
Key Features:
- High specificity: Selective photostimulation of corticospinal neurons expressing ChR2.
- Precision tools: Use of laser scanning technology with real-time power control.
- Affordable setup: Inexpensive video motion tracking with high-speed cameras.
- Advanced analysis: MATLAB-based motion tracking for accurate movement characterization.
This approach represents a significant advancement in optogenetic motor mapping, combining precision and accessibility for neuroscience research.
Presentation: 11th National Congress of the Belgian Society for Neuroscience
Title:
Striatopallidal NMDA-Receptors Control Locomotor and Conditioning Behaviors
Conference Date: May 22, 2015
Location: Mons, Belgium
Presentation Type: Oral or Poster
Authors:
L. Lambot¹, E. Chaves Rodriguez¹, Y. Li², S.N. Schiffmann¹, A. De Kerchove D’Exaerde¹, D. Gall¹
¹Université Libre de Bruxelles, Faculté de Médecine, Belgium
²University of Florida, College of Medicine, Department of Neurology, United States
Abstract:
The basal ganglia (BG) are critical for goal-directed action selection, motor control, and habit formation. Within the BG, the striatum plays a central role, comprising medium spiny neurons (MSNs) that project into two distinct pathways: the direct pathway from striatonigral MSNs (dMSNs) and the indirect pathway from striatopallidal MSNs (iMSNs). Balanced activity between these pathways is essential for normal BG functions, and their disruption is implicated in neuropsychiatric disorders such as Parkinson’s and Huntington’s diseases and drug addiction.
While the importance of striatal NMDA receptors (NMDA-R) in BG physiology is well established, their specific role in striatopallidal neurons remains unclear. This study addressed this question by conditionally deleting the GluN1 subunit of NMDA-R specifically in iMSNs (cKO).
Key Findings:
- At the cellular level, GluN1 deletion resulted in fewer and weaker corticostriatopallidal synapses. Intrinsically, iMSNs exhibited increased electro-responsiveness, a homeostatic adaptation compensating for reduced synaptic inputs.
- Behaviorally, the absence of NMDA-R in iMSNs led to aberrant action selection in goal-directed behavior, altered motor strategies (despite intact motor learning), and disrupted habituation and amphetamine locomotor sensitization.
These findings indicate that striatopallidal NMDA-Rs are critical for operant and habituation behaviors, underscoring their role in maintaining BG functionality and balanced motor control.
Acknowledgments:
Funding:
- FRIA PhD Fellowship, FRS-FNRS (Belgium)
- Van Buuren Grant, Fonds David et Alice Van Buuren
- Fondation Médicale Reine Elisabeth (Belgium)
Special Thanks:
Dr. Frédéric Bollet-Quivogne, Light Microscopy Facility (LiMiF)
Keywords:
NMDA receptor, Neuronal morphology, Striatum, Goal-directed behavior, Synaptic transmission
Citation:
Lambot L, Chaves Rodriguez E, Li Y, Schiffmann SN, De Kerchove D’Exaerde A, and Gall D (2015). Striatopallidal NMDA-receptors control locomotor and conditioning behaviours. Front. Neurosci. Conference Abstract: 11th National Congress of the Belgian Society for Neuroscience. doi: 10.3389/conf.fnins.2015.89.00074.
Frontiers _ Striatopallidal NMDA-receptors control locomotor and conditioning behaviours_
Presentation: 11th National Congress of the Belgian Society for Neuroscience
Title:
Functional Role of PDGFRβ, a Striatopallidal-Specific Gene, in Motor and Motivational Behavior
Conference Date: May 22, 2015
Location: Mons, Belgium
Presentation Type: Poster
Authors:
D. Karadurmus¹, S.L. Ena¹, L. Lambot¹, A. De Kerchove D’Exaerde¹, S.N. Schiffmann¹
¹Université Libre de Bruxelles, ULB Neuroscience Institute, Belgium
Abstract:
The basal ganglia (BG), a network of interconnected nuclei, play a central role in motor control and motivation. The striatum, the primary input of the BG, consists mainly of medium spiny neurons (MSNs), which are subdivided into striatopallidal (STP) and striatonigral (STN) neurons. These subpopulations form the indirect and direct BG pathways, respectively, exerting opposing effects on motor and motivational behaviors. Understanding the cellular mechanisms of these pathways is crucial for disorders such as Huntington’s and Parkinson’s diseases and addiction.
Our laboratory previously identified gene expression profiles specific to STN and STP neurons using microarray analysis (Ena, 2013). This project investigates the functional roles of STP- or STN-specific genes in locomotor control and addiction behaviors. By selectively repressing target genes using floxed mice or lentivirus-mediated shRNA interference, we perform phenotypic analyses through behavioral tests. Molecular biology and electrophysiology are employed to uncover mechanisms underlying observed phenotypes.
We focused on PDGFRβ, a gene enriched in STP neurons with potential functional importance in the striatum. STP-specific expression of PDGFRβ was validated, and its activation pathway in STP neurons was explored in primary striatal cultures. A mouse model with PDGFRβ repression in striatal neurons is being developed to study its effects in vivo.
Key Findings:
- Validated PDGFRβ as a gene specifically expressed in STP neurons.
- Explored the PDGFRβ activation pathway in primary striatal cultures.
- Ongoing generation of a mouse model to investigate PDGFRβ deletion effects on motor and motivational behaviors.
Acknowledgments:
Funding:
- FRIA PhD Fellowship, FRS-FNRS (Belgium)
- Fondation Médicale Reine Elisabeth (Belgium)
Keywords:
Striatum, PDGFRβ, Striatopallidal pathway, Striatonigral pathway, Cell-type-specific gene
References:
Ena, S.L., De Backer, J.F., Schiffmann, S.N., De Kerchove D’Exaerde, A. (2013). FACS array profiling identifies Ecto-5′ nucleotidase as a striatopallidal neuron-specific gene involved in striatal-dependent learning. J Neurosci 33, 8794-8809. DOI: 10.1523/JNEUROSCI.2989-12.2013
Citation:
Karadurmus D, Ena SL, Lambot L, De Kerchove D’Exaerde A, and Schiffmann SN (2015). Functional Role of PDGFRβ, a Striatopallidal-Specific Gene, in Motor and Motivational Behavior. Front. Neurosci. Conference Abstract: 11th National Congress of the Belgian Society for Neuroscience. DOI: 10.3389/conf.fnins.2015.89.00045
Frontiers _ Functional role of PDGFRb, a striatopallidal specific gene, in motor and motivational behavior
Presentation: Biophysical Society 58th Annual Meeting
Title:
Distinct Target Selectivity of Fast-Spiking Interneurons in the Regulation of Striatal Output Pathways
Conference Date: January 2014
Location: San Francisco, CA, USA
Presentation Type: Poster (Board B665, Session 1935-Pos)
Authors:
L. S. Lambot¹, S. N. Schiffmann², D. Gall²
¹University of Chicago
²Université Libre de Bruxelles, Brussels, Belgium
Abstract:
The striatum, the primary input nucleus of the basal ganglia (BG), integrates excitatory inputs from the cortex and thalamus to regulate motor control and movement. Medium spiny neurons (MSNs), the principal neuronal type in the striatum, are divided into two subpopulations:
- Direct pathway MSNs (dMSNs): Facilitate movement via the “direct pathway.”
- Indirect pathway MSNs (iMSNs): Suppress movement via the “indirect pathway.”
Balanced activity between these pathways is critical for proper motor function, and disruptions are associated with movement disorders such as Parkinson’s disease and drug addiction. The activity of MSNs is regulated by feedforward inhibition mediated by parvalbumin-expressing fast-spiking interneurons (FSIs). FSIs inhibit both dMSNs and iMSNs and are interconnected through electrical and chemical synapses, playing a pivotal role in shaping striatal output.
This study investigates the target selectivity of FSIs in regulating dMSNs and iMSNs using a combination of optogenetic and electrophysiological techniques:
- Optogenetics: FSIs are selectively stimulated with 470 nm blue light.
- Electrophysiology: Patch-clamp recordings in acute brain slices measure inhibitory post-synaptic currents (IPSCs) in both MSN subpopulations.
Key Findings:
- FSIs differentially regulate direct and indirect pathway MSNs, contributing to the balance of striatal output.
- The effects of chronic amphetamine/cocaine administration on FSI-MSN microcircuitry were examined, providing insights into pathological states.
This work enhances our understanding of striatal microcircuits and their role in both physiological and pathological motor behaviors.
Key Features:
- Techniques: Combination of optogenetics and electrophysiology for precise FSI stimulation and MSN recording.
- Focus: Differential effects of feedforward inhibition on dMSNs and iMSNs.
- Pathological Models: Analysis of changes in microcircuitry under chronic drug exposure.
Citation:
Lambot L, Schiffmann SN, Gall D. Distinct Target Selectivity of Fast-Spiking Interneurons in the Regulation of Striatal Output Pathways. Biophysical Journal 106(2):383a. DOI: 10.1016/j.bpj.2013.11.2168
Distinct Target Selectivity of Fast-Spiking Interneurons in the Regulation of Striatal Output Pathways_ Biophysical Journal
Media Coverage
Science Translational Medicine, Published on 2017-02-24
The Big Picture: Our team’s work on Alzheimer’s disease, utilizing human neurons transplanted into a mouse model, has been recognized as an Editor’s Choice by Science Translational Medicine. This recognition from Science Translational Medicine, a journal at the crossroads of multiple scientific disciplines, highlights the wide-reaching implications of our Alzheimer’s research and its promise in addressing complex neurological diseases.
Abstract: Human neurons transplanted into a mouse model for Alzheimer’s disease show human-specific vulnerability to β-amyloid plaques and may help to identify new therapeutic targets.

La Libre, Belgium, Published on 2017-02-24 at 10:58
La Libre featured our team’s work on Alzheimer’s disease, achieved in collaboration with KU Leuven. We transplanted human neurons into mouse brains with amyloid plaques, uncovering the human neurons’ increased sensitivity to Alzheimer’s pathology. This breakthrough paves the way for deeper molecular understanding and targeted therapeutic development.
Read the article here | Translation here

The Big Picture:
Science Daily highlighted our Alzheimer’s research for enabling the direct study of human cell pathology in mouse models. This advancement allows researchers to identify drug targets within human neurons, something not feasible in earlier models.
Drug Target Review, Published in 2017
The Big Picture:
This feature emphasized how our innovative model accelerates Alzheimer’s research by enabling direct observation of disease processes in human neurons. The approach significantly enhances the pace and precision of discovering drug targets for Alzheimer’s treatment.
Nature Reviews Neurology, Published in 2017
The Big Picture:
Nature Reviews Neurology praised our Alzheimer’s model for its capacity to replicate human-specific disease hallmarks, such as neurite dystrophy and tau pathology, at an unprecedented scale. These findings address limitations of previous models and advance Alzheimer’s research.
KUL News, Published in 2017
The Big Picture:
KU Leuven celebrated the research for allowing precise observation of Alzheimer’s pathology in human brain cells. This innovative technique opens new doors to understanding the relationship between amyloid and tau proteins, advancing the holy grail of Alzheimer’s research.
Electronics Specifier, Published in 2017
The Big Picture:
Electronics Specifier spotlighted the work for its novel approach in characterizing Alzheimer’s processes, offering valuable insights into the disease mechanisms at play in human patients.
Focus on Belgium, Published in 2016
This feature covered my PhD research on the NMDA receptor’s role in Parkinson’s disease. By highlighting synaptic and motor disruptions caused by NMDA receptor dysfunction, the study sheds light on procedural memory and addiction-related behaviors, advancing our understanding of basal ganglia circuits.
Read the article here | Translation here

ULB News, Belgium, on May 9, 2016
The Big Picture: This press release highlights research conducted at the ULB Neuroscience Institute, showcasing the pivotal role of NMDA glutamate receptors in procedural memory and motor control. The study emphasized the receptor’s impact on synaptic plasticity in basal ganglia circuits, elucidating how disruptions can contribute to neurological conditions like Parkinson’s disease and addiction. The findings provide a deeper understanding of the cellular and systemic mechanisms underlying these pathologies.
Read the article here | Translation here

Blog
My Diary
13 Aug 2025